Ovarian cancer remains one of the most formidable challenges in oncology, particularly the high-grade serous ovarian carcinoma (HGSOC), which is notorious for its late detection and aggressive nature. Current understanding indicates that a significant proportion of these cancers may originate not from the ovaries themselves but from the fallopian tubes. Recent research involving mice offers promising insights that may change early detection methodologies and therapeutic strategies for HGSOC in humans.
A wealth of research over the past decade has led scientists to consider the fallopian tubes as potential culprits in the development of many ovarian cancers. These structures have increasingly been identified as the sites where precursors to HGSOC can develop. However, a crucial knowledge gap still exists regarding the specific cell types in the fallopian tubes that initiate these malignancies. The challenge lies in detecting HGSOC early enough to intervene effectively; at present, most diagnoses occur at a late stage, severely limiting treatment options and leading to poor prognoses.
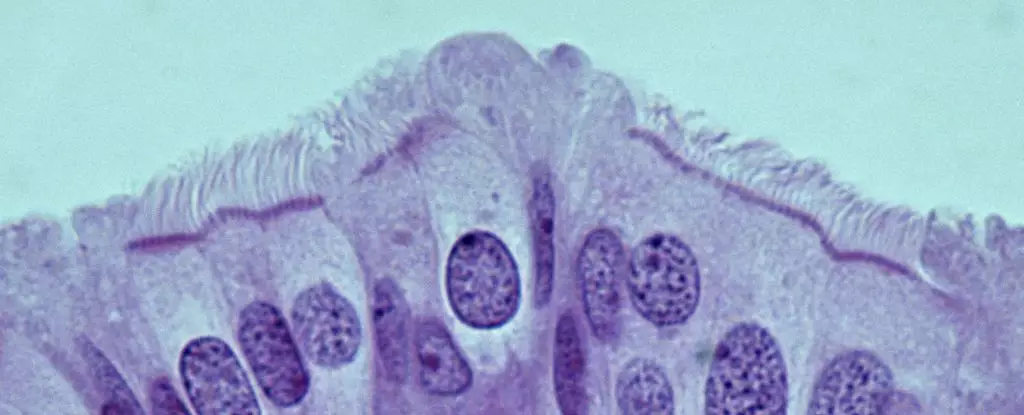
Recently, a team led by Cornell University pathologist Alexander Nikitin has changed the landscape of ovarian cancer research by meticulously mapping the cellular architecture of the mouse oviducts. This study outlines the fundamental types of cells present, providing a critical baseline for understanding which of these might be implicated in cancer development. The identification of these cell types paves the way for potential diagnostic markers crucial for early detection.
The findings from Nikitin’s research are particularly noteworthy because they challenge earlier assumptions that stem cells in the ovaries carry the primary risk of leading to HGSOC. Instead, the research highlights the significance of pre-ciliated cells in the oviducts. These transitional cells, which play a role in facilitating the movement of oocytes along the reproductive tract, demonstrate an unexpected susceptibility to cancer when certain genetic mutations associated with HGSOC are present.
Mutation interactions within these pre-ciliated cells can create a conducive environment for tumor formation. Nikitin’s team discovered that when key mutations linked to HGSOC influence the behavior of these cells, they lead to a markedly efficient process of cancer development. This correlation hints at a possible mechanistic bridge between mucosal cell health—specifically concerning cilia formation—and the incidence of ovarian cancer, a revelation that could compel further investigation.
What renders these findings even more compelling is their resonance beyond ovarian cancer, as the dysregulation of ciliary formation is implicated in other types of malignancies, such as pancreatic cancer. The interplay between various genetic alterations and their physiological consequences poses a rich avenue for exploration in oncological research. The realization that the mechanisms driving various cancers may share a common path could lead to more comprehensive cancer treatment strategies that address underlying cellular dysfunctions instead of merely targeting the tumors themselves.
As we stand on the brink of potentially transformative discoveries in early diagnostic techniques for HGSOC, the implications of this study cannot be overstated. If the findings regarding mice translate effectively to humans, they could revolutionize the way we approach ovarian cancer screening and therapy. This scenario not only holds promise for improved patient survival rates but also enhances our overall understanding of cancer biology, laying the groundwork for future research aimed at early intervention.
While the implications of this research are profound, they also underscore the necessity for continued exploration into the cellular mechanisms that foster ovarian carcinogenesis. Further studies will be essential in deciphering whether other genetic factors associated with HGSOC present similar vulnerabilities in cell types found in human fallopian tubes. Identifying these pathways is crucial for developing targeted therapies and diagnostic tools that could ultimately save countless lives.
The recent research conducted on mice presents a paradigm shift in our understanding of ovarian cancer origins and highlights the need for innovative approaches to early detection. By delving deeper into the characteristics and behaviors of specific cell types within the fallopian tubes, researchers may finally be able to unlock the secrets of HGSOC and emerge victorious in the ongoing battle against this deadly disease.

Leave a Reply