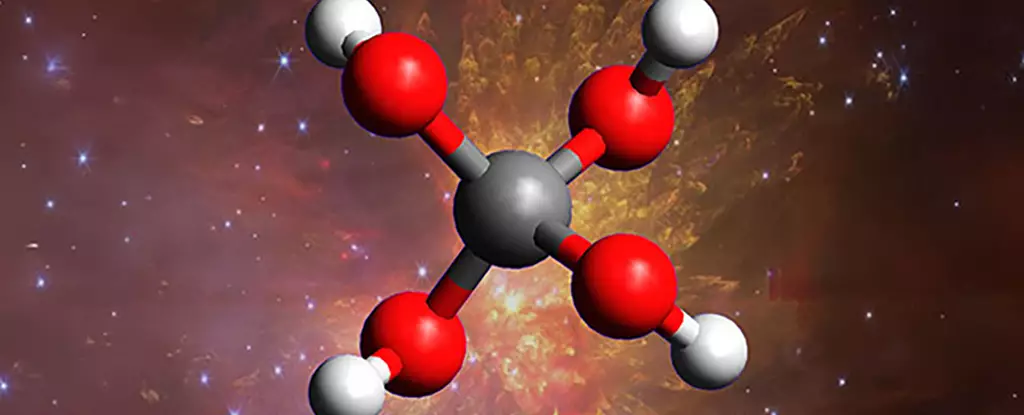
In a striking leap forward, scientists have confirmed the existence of methanetetrol, an extraordinarily unstable molecule often dubbed a “super alcohol,” produced under extreme simulated space conditions. This discovery unveils a radical new layer of complexity within the cosmic chemical landscape, casting doubt on long-held notions that space is a relatively inert environment composed mainly of simple molecules. Instead, the findings suggest that the universe might be teeming with exotic, reactive compounds capable of shaping the very fabric of celestial phenomena.
The implications are profound. For decades, astrophysicists operated on the assumption that the harsh, frigid environments of interstellar clouds limited chemistry to basic molecules like water and simple hydrocarbons. Yet, the formation of a molecule like methanetetrol—comprising four hydroxyl groups bound to a single carbon—defies previous expectations. This molecule’s extraordinary instability means it exists only fleetingly, but its very creation in laboratory conditions raises the question: what other “impossible” molecules are lurking in the depths of space?
This discovery signals a paradigm shift, hinting that the cosmos may host a far richer chemical diversity than previously imagined. It challenges the assumption that complex molecules can only form in warm, planetary environments, forcing a re-evaluation of models related to star formation, planetary chemistry, and potentially, the origins of life itself. If such molecules can appear under the extreme cold and radiation conditions of space, what does this suggest about the potential for prebiotic chemistry in regions once thought sterile?
Bridging the Gap Between Theory and Reality: The Limitations of Earthbound Detection
Detecting methanetetrol directly in space remains a formidable challenge. Its inherent fragility means it disintegrates rapidly when exposed to light, a process known as dissociative photoionization, which hampers observational efforts. Scientists are left with only fleeting glimpses, relying heavily on simulated experiments and the indirect signatures they produce. This highlights a critical gap: our current instrumentation and understanding may be insufficient to unveil the full spectrum of space chemistry.
While laboratory simulations provide valuable insights, they cannot fully replicate the chaotic and unpredictable conditions of the cosmos. The fleeting nature of methanetetrol reminds us of our limitations in detecting the more ephemeral constituents of the universe. It also emphasizes the importance of developing more sensitive, robust tools in spectroscopy and telescope technology, capable of capturing these transient phenomena amid the vast emptiness of space.
Moreover, the phenomenon underscores a fundamental flaw in our approach to cosmic chemistry—our tendency to underestimate the universe’s ability to generate complex, unstable molecules in its coldest, most hostile regions. These molecules could serve as catalysts or building blocks for more stable compounds, directly influencing the pathways of star formation and planetary development, potentially seeding planets with the raw materials for life.
Redefining Cosmic Possibilities: What Does This Mean for Life Beyond Earth?
From a broader perspective, the existence of molecules like methanetetrol stirs a radical reconsideration of astrobiological potential. If space can forge these “impossible” molecules, then perhaps life-building blocks are more commonplace and resilient than previously thought. This shifts the conversation from a narrow focus on Earth-like conditions to a more inclusive view of the universe’s capacity to nurture complexity beyond our current understanding.
Such revelations bolster the argument that the origins of life could be far more prevalent, seeded by molecules that previously went unnoticed due to their fleeting existence or the limitations of detection technology. They suggest a universe where chemistry is an active, dynamic player—an environment where the building blocks of life can emerge in surprising forms and contexts.
Politically and socially, this advances the conversation about space exploration and the importance of continued investment. Instead of viewing space science as a speculative endeavor, we must recognize it as central to understanding humanity’s place in the cosmos. The possibility that the universe teems with complex, transient chemicals challenges us to rethink funding priorities, educational focus, and international collaboration in space research.
Ultimately, the discovery of methanetetrol serves as both a wake-up call and an inspiration. It pushes us to acknowledge that our knowledge of the universe’s chemical diversity is just beginning—and that by expanding our reach, embracing innovative techniques, and daring to explore the unknown, we may uncover truths that fundamentally alter our understanding of reality itself.

Leave a Reply